Titration Concentration Of Solution . Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. \ce{naoh}\) is required to reach the end point when titrated. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. The known solution (titrate) is added in. Suppose that a titration is performed and \(20.70 \: Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs.
from www.chegg.com
Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. The known solution (titrate) is added in.
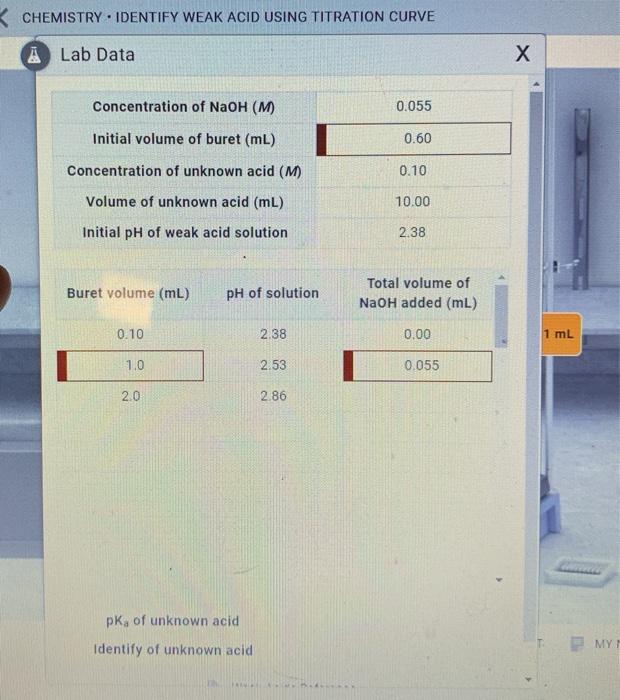
Solved CHEMISTRY. IDENTIFY WEAK ACID USING TITRATION CURVE A
Titration Concentration Of Solution Suppose that a titration is performed and \(20.70 \: A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The known solution (titrate) is added in. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \: Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown.
From chem.libretexts.org
4.5 Concentration of Solutions Chemistry LibreTexts Titration Concentration Of Solution A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume. Titration Concentration Of Solution.
From www.chegg.com
Solved CHEMISTRY. IDENTIFY WEAK ACID USING TITRATION CURVE A Titration Concentration Of Solution Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume. Titration Concentration Of Solution.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Concentration Of Solution \ce{naoh}\) is required to reach the end point when titrated. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is the slow addition of one solution of. Titration Concentration Of Solution.
From socratic.org
How to calculate the concentration of the acid solutions? Socratic Titration Concentration Of Solution Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. The known solution (titrate) is added in. A titration is a laboratory technique used to precisely measure molar concentration. Titration Concentration Of Solution.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Concentration Of Solution The known solution (titrate) is added in. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is a technique of determining the concentration of an unknown solution. Titration Concentration Of Solution.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration Titration Concentration Of Solution The known solution (titrate) is added in. \ce{naoh}\) is required to reach the end point when titrated. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Suppose that a titration is performed and \(20.70 \: Titration is a technique of determining the concentration of an unknown solution by using. Titration Concentration Of Solution.
From mungfali.com
Acid Base Titration Procedure Titration Concentration Of Solution A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. A titration is a technique where a solution of known concentration is used to determine the concentration of an. Titration Concentration Of Solution.
From mungfali.com
Acid Base Titration Calculation Titration Concentration Of Solution Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. \ce{naoh}\) is required to reach the end point when titrated. The known solution (titrate) is added in. Titration. Titration Concentration Of Solution.
From mssmithsechs.weebly.com
Acids, Bases, Solutions, and Titrations MS. SMITH'S CLASS Titration Concentration Of Solution Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. The known solution (titrate) is added in. A titration is a technique where a solution of known concentration is. Titration Concentration Of Solution.
From opentextbc.ca
14.7 AcidBase Titrations Chemistry Titration Concentration Of Solution Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. Suppose that a titration is performed and \(20.70 \: Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique. Titration Concentration Of Solution.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download Titration Concentration Of Solution \ce{naoh}\) is required to reach the end point when titrated. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. The known solution (titrate) is. Titration Concentration Of Solution.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution Titration Concentration Of Solution Suppose that a titration is performed and \(20.70 \: The known solution (titrate) is added in. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. \ce{naoh}\) is required to reach the end point when titrated. Titration is the slow addition of one solution of a known concentration (called. Titration Concentration Of Solution.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Titration Concentration Of Solution Suppose that a titration is performed and \(20.70 \: Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is a technique of determining the concentration of. Titration Concentration Of Solution.
From labthink.netlify.app
What is titration and how does it work Titration Concentration Of Solution Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. \ce{naoh}\) is required to reach the end point when titrated. A titration is a technique where a solution of. Titration Concentration Of Solution.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Titration Concentration Of Solution Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a technique where a solution of known concentration is used to determine the concentration of an. Titration Concentration Of Solution.
From saylordotorg.github.io
AcidBase Titrations Titration Concentration Of Solution A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. \ce{naoh}\) is required to reach the end point when titrated. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Suppose that a titration is performed. Titration Concentration Of Solution.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Titration Concentration Of Solution Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. The known solution (titrate) is added in. Suppose that a titration is performed and \(20.70 \: Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown.. Titration Concentration Of Solution.
From www.chegg.com
TitrationStandardization of Sodium Hydroxide Titration Concentration Of Solution A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. The known solution (titrate) is added in. Suppose that a titration is performed and \(20.70 \: Titration is the. Titration Concentration Of Solution.